Advanced MS therapeutics combined with the innovative NeuroQuant® software provides invaluable information to neurologists and radiologists.
(SAN DIEGO) April 21, 2015 – Cortechs.ai, the leading medical software innovator providing solutions for quantitative brain volume analysis is pleased to announce a partnership agreement with Novartis Pharma AG, a global pharmaceutical company. The companies will collaborate to develop NeuroQuant’s powerful brain volume quantification report targeting the identification, measurement and tracking of brain volume loss in multiple sclerosis patients.
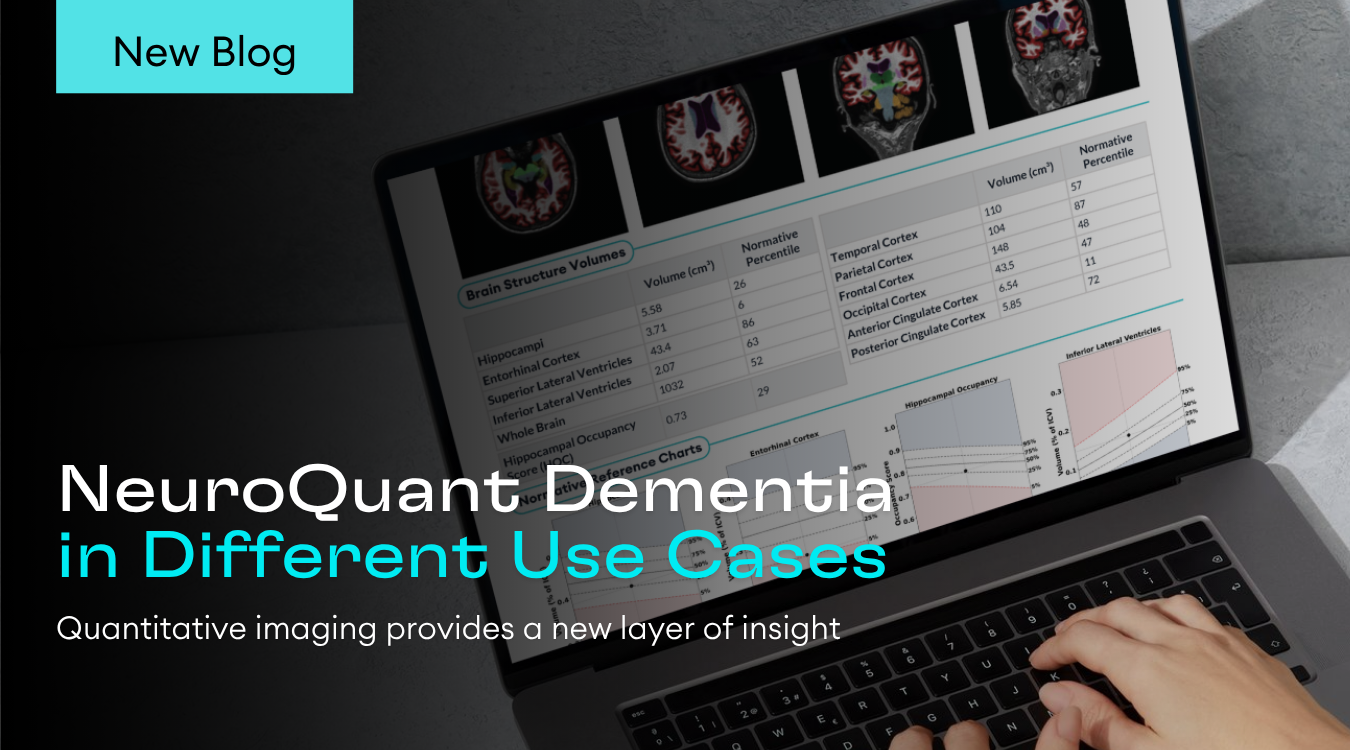
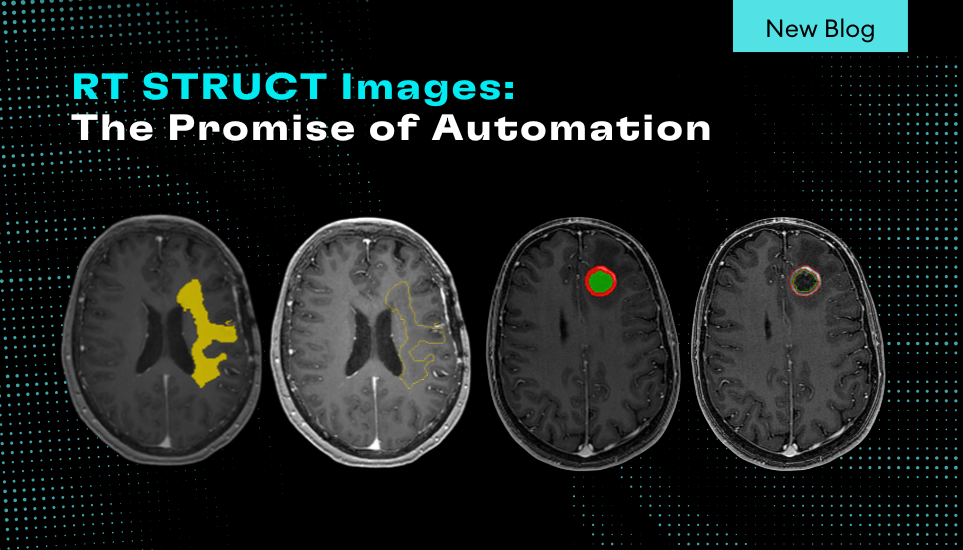
The resulting report enables clinicians to automatically conduct accurate and consistent measurements of the whole brain, left and right thalami and the lateral ventricles out of 3D T1 MRI images. The report is targeting the identification and tracking of neurodegeneration in these sub-cortical structures. This breakthrough solution, which is available both online and as an installed software solution, provides neurologists and radiologists with objective support of brain volume loss and its changes over time and as compared to normative values.
“We’re pleased to partner with Novartis to advance the clinical care and assessment of multiple sclerosis worldwide,” said Guri Stark, Cortechs.ai’ CEO. “We believe this partnership will empower clinicians with objective quantitative data, while continuing NeuroQuant’s growth momentum. This, in-turn furthers our vision to establish NeuroQuant as the standard measurement solution for all brain disorders, including Alzheimer’s disease, epilepsy, multiple sclerosis and traumatic brain injury.”
About Cortechs.ai
Cortechs.ai develops and markets cutting-edge brain imaging solutions used by neurologists and radiologists in hundreds of clinics and research centers around the world. CorTechs’ flagship product, NeuroQuant®, is a breakthrough, 510(k) cleared software medical device that makes quantitative analysis of MRI images of the human brain a routine part of clinical practice. As the first FDA cleared and CE marked medical device capable of automatically detecting and quantifying atrophy in the human brain, NeuroQuant® brings sophisticated, accurate, and fully automated MRI post-processing capabilities to the physician’s desktop or mobile device. This provides neurologists, radiologists, and clinical researchers with a convenient and cost-effective means to quantify atrophy of brain structures to help diagnose a variety of brain disorders, including conditions such as Alzheimer’s disease, epilepsy, multiple sclerosis and traumatic brain injury. Please visit cortechs.ai or www.wholebrainatrophy.com for further information.
###
Media Contact:
Travis Foegler
Phone: +1 (619) 450-9094
Email: Tfoegler@cortechs.ai