- NeuroQuant® for ARIA
Automated quantification, visualization, and longitudinal tracking of brain lesions to support ARIA surveillance
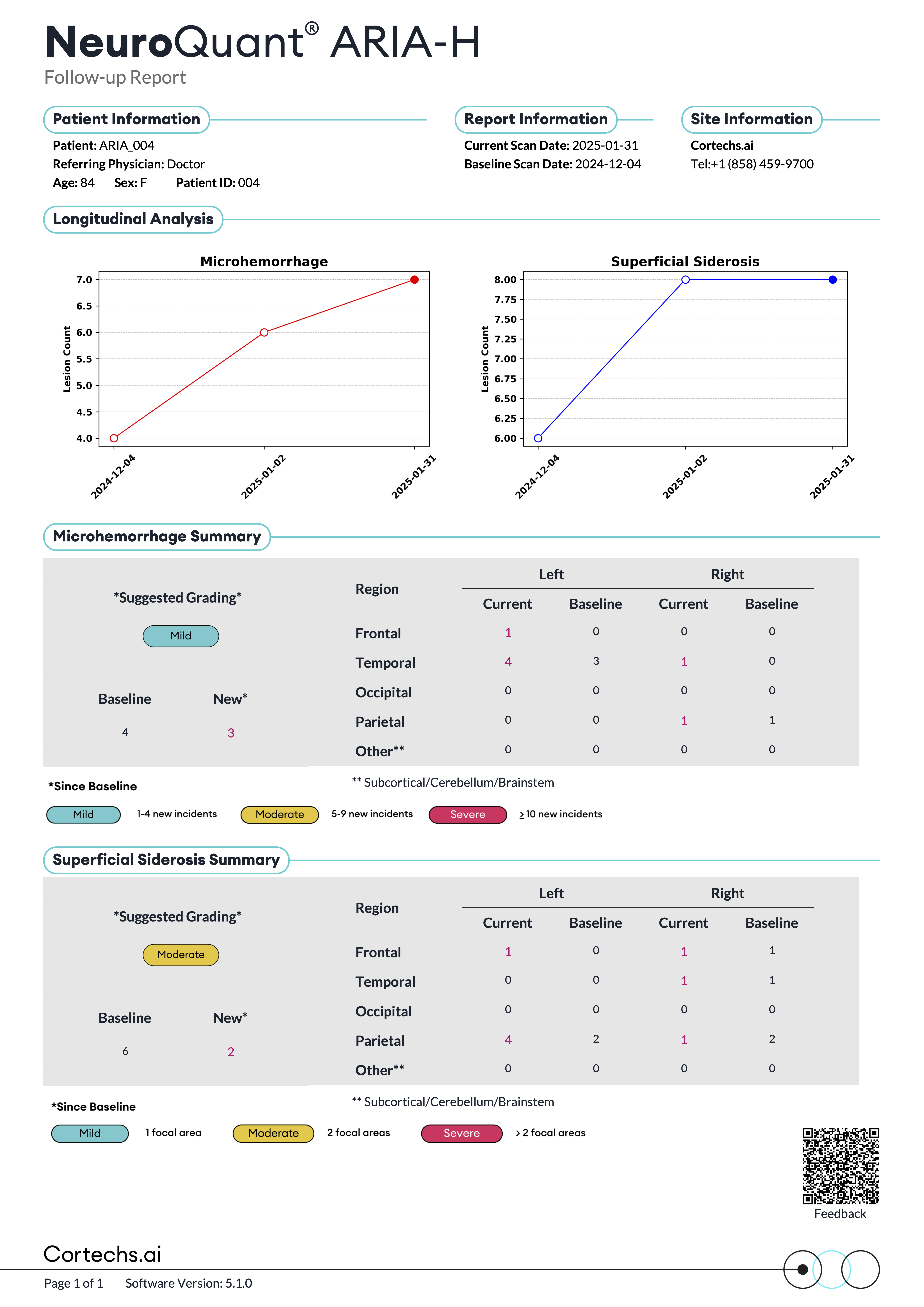
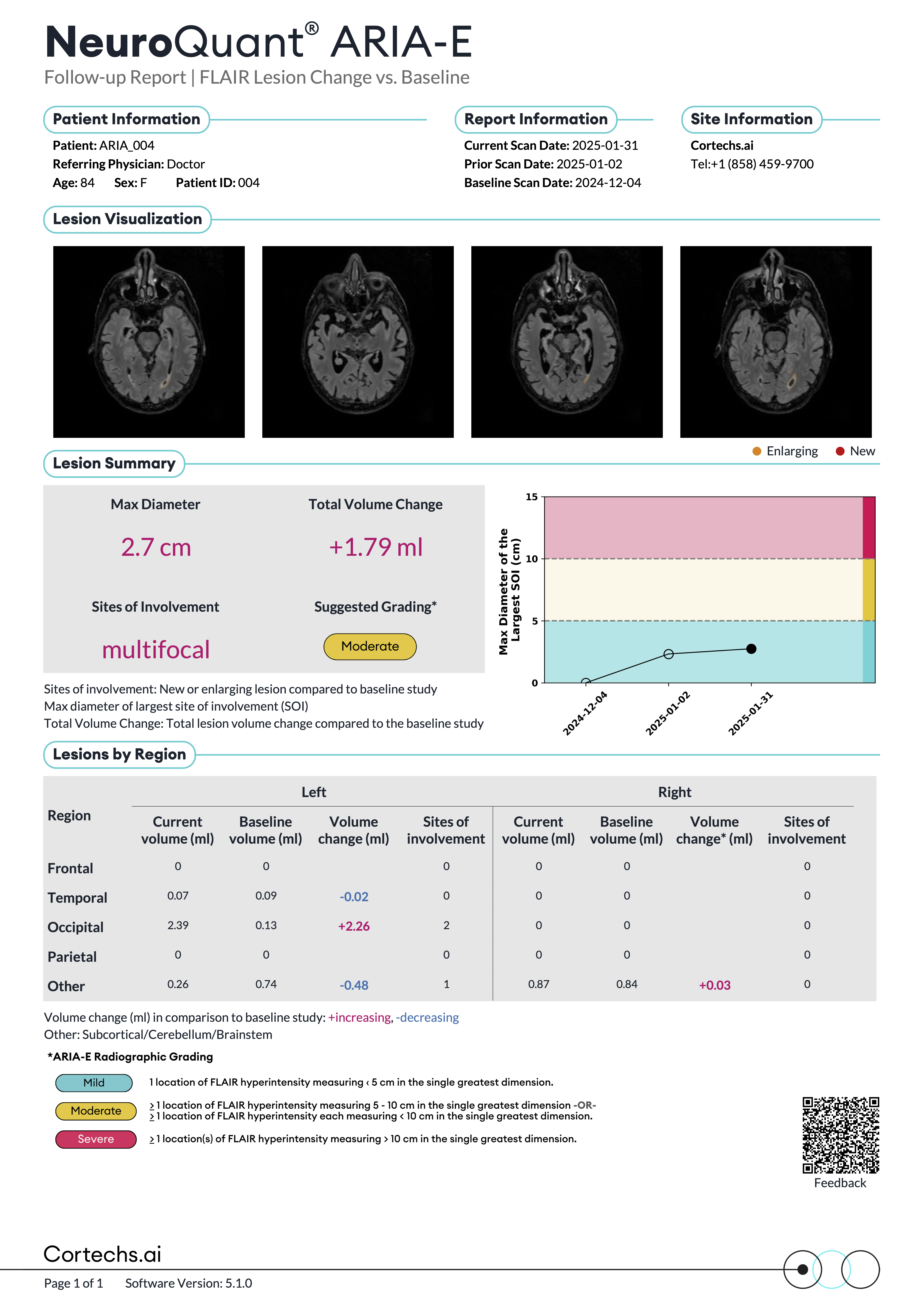
NeuroQuant® v5 offers precise segmentation, quantification, and longitudinal tracking of both FLAIR and T2*GRE/SWI lesions. This advanced functionality offers assistance with the surveillance of amyloid-related imaging abnormalities, such as ARIA-E and ARIA-H, in patients receiving anti-amyloid therapies.
A Complete Solution for Quantitative Lesion Tracking
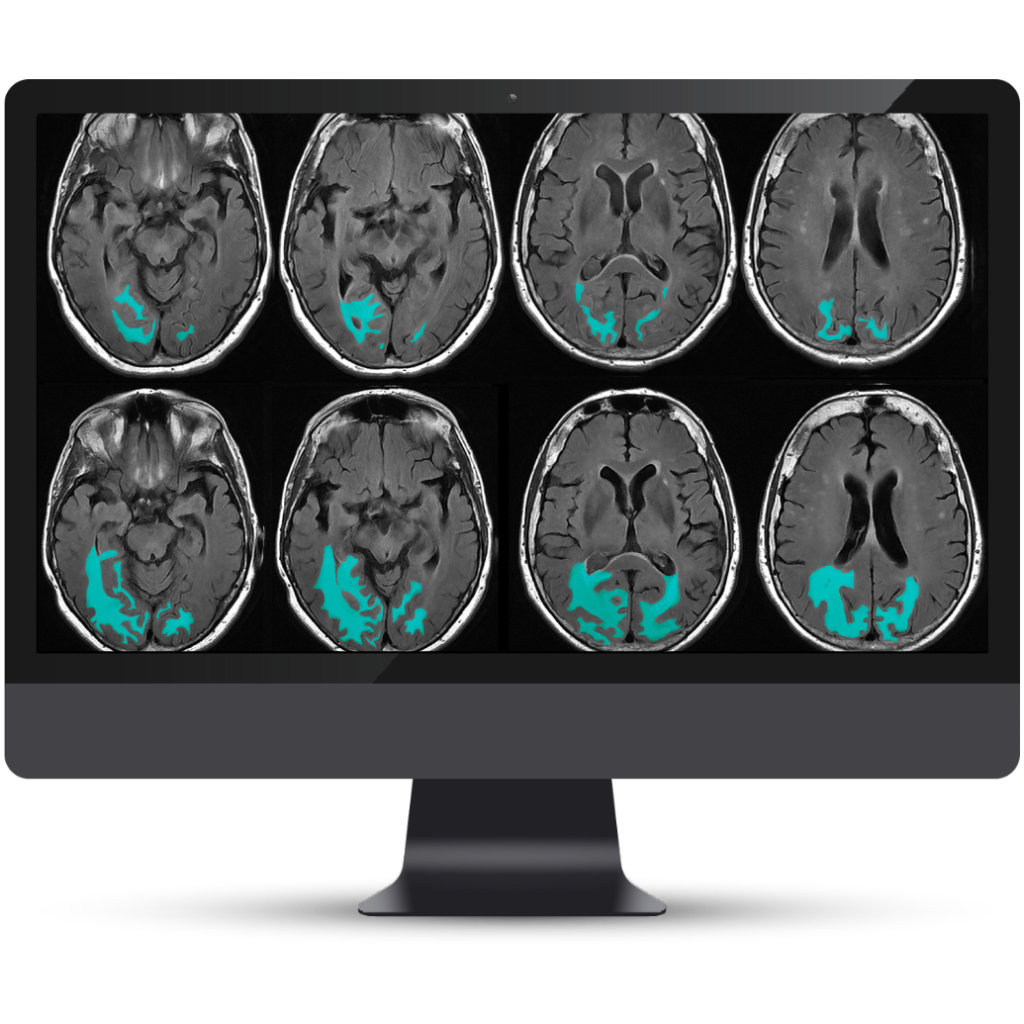
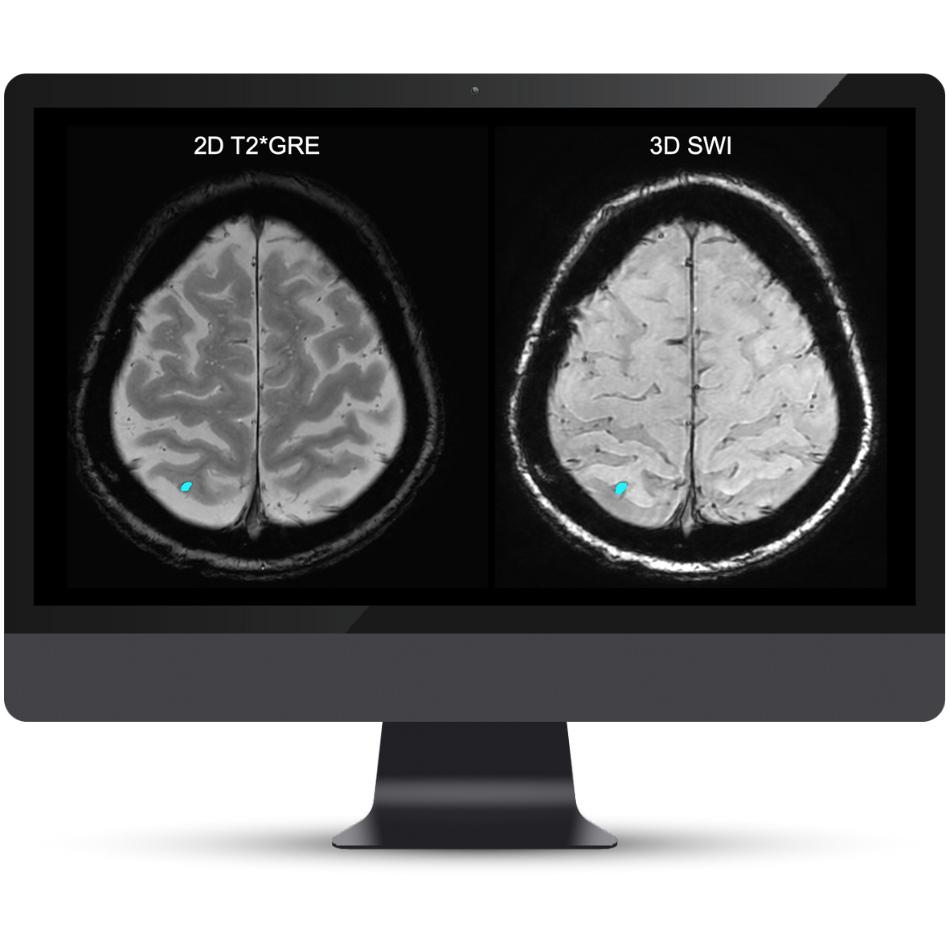
NeuroQuant® v5 is the only clinically validated solution that detects and quantifies cerebral microbleeds and superficial siderosis on both T2*GRE and SWI scans. Coupled with FLAIR lesion quantification of cerebral edema, NeuroQuant® v5 offers a complete solution for AI-assisted monitoring of Alzheimer’s Disease patients on anti-amyloid immunotherapies.
Benefits
Lesion quantification
Accurate segmentation and longitudinal tracking of FLAIR, T2*GRE, and SWI lesions
OEM protocol standardization
To ensure reproducible outcomes and accuracy over time
Longitudinal disease tracking
Monitor lesion counts and maximum diameters over time, with comparisons to baseline and prior timepoints
NeuroQuant® ARIA reports
Reports that provide quantitative insights to support physicians in tracking FLAIR and T2*GRE/SWI lesions over time compared with a baseline study.

Understanding CPT Codes 0865T & 0866T
As of January 1, 2024, new Category III CPT codes (0865T, 0866T) are active for AI-assisted quantitative brain MRI analysis. These codes apply to a variety of vendor solutions, including Cortechs.ai, and serve as an essential step toward achieving permanent reimbursement.