What an amazing and exhilarating first half of 2016!
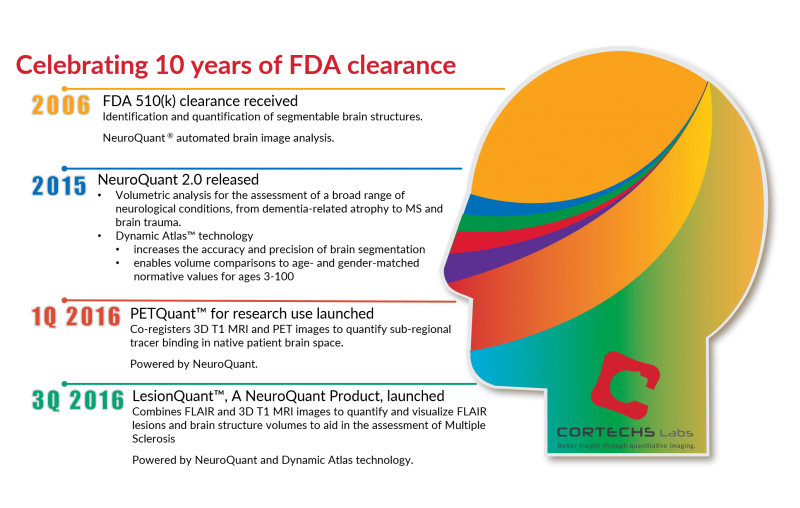
August 10, 2016 marked 10 years of FDA 510(k) clearance for our flagship product, NeuroQuant®. The clinical release of NeuroQuant has advanced and revolutionized the quality of patient care by providing physicians with objective, automated, precise and reliable measures of subcortical brain structure volumes in about 5 minutes.
Since FDA clearance in 2006, NeuroQuant has been warmly received by the medical community. In fact, since 2006, roughly 115,000 NeuroQuant reports have been generated worldwide, and that number continues to grow. It is truly remarkable to see how many hospitals, clinics, imaging facilities, universities and military hospitals have recognized and continue to recognize the importance of quantitative imaging in clinical practice and in research.
The visionary team here at Cortechs.ai has been on fire and continues to drive their passion and commitment to developing inspired and relevant products to aid physicians achieve superior clinical insight. The past six months have been incredible and the Cortechs.ai team has worked tirelessly to release new products, obtain additional regulatory licensing, and develop new strategic partnerships, as well as, formally expand into Europe.
In January, we finalized our collaboration with GE Healthcare to make NeuroQuant available in the GE Health Cloud Ecosystem, enabling NeuroQuant to be fully integrated into GE Health Cloud’s clinical workflow.
In February, we were pleased to receive Health Canada/Santé Canada Medical Device License for marketing and distribution of NeuroQuant in Canada.
In March, we launched PETQuant™, a research-only tool, that co-registers 3D T1 MRI and PET imaging to provide post-acquisition analysis of PET brain studies to quantify subregional tracer binding in native patient brain space.
In May, we announced a partnership agreement with Olea Medical, to offer NeuroQuant in Olea Sphere, a suite of innovative imaging applications.
Also in May, we revealed that we accelerated our international expansion into the European market to address growing demand and support our existing customer base within Europe.
Finally, in August, we released the highly anticipated LesionQuant™, a NeuroQuant product, for FLAIR lesion identification, segmentation, quantification and visualization. This significant enhancement to NeuroQuant is intended to aid physicians in disease progression and monitoring of Multiple Sclerosis by combining information from 2D or 3D FLAIR and 3D T1 MR images, providing lesion and brain structure quantification in a completely automated and optimized easy-to-read report.
With these expanded capabilities and partnerships, NeuroQuant continues to be the paramount solution for the assessment of neurological conditions. From brain development to Alzheimer’s disease, multiple sclerosis, epilepsy and brain trauma, NeuroQuant helps enhance the quality of patient care.
It’s incredible to think of all the impressive changes that have taken place at Cortechs.ai over the past 10 years, since NeuroQuant received FDA clearance, not to mention, during just the first half of 2016. More exciting developments are planned for the second half and we look forward to sharing them with you as the excitement continues.
Sincerely,
Guri Stark
CEO, Cortechs.ai
Like this post? You might also find these interesting.
- A Word from the CEO: 2015 in Review
- Welcome to the Cortechs.ai Future: Mid-Year Review from our CEO Guri Stark
- A Word from the CEO: 2014 in Review
![]()