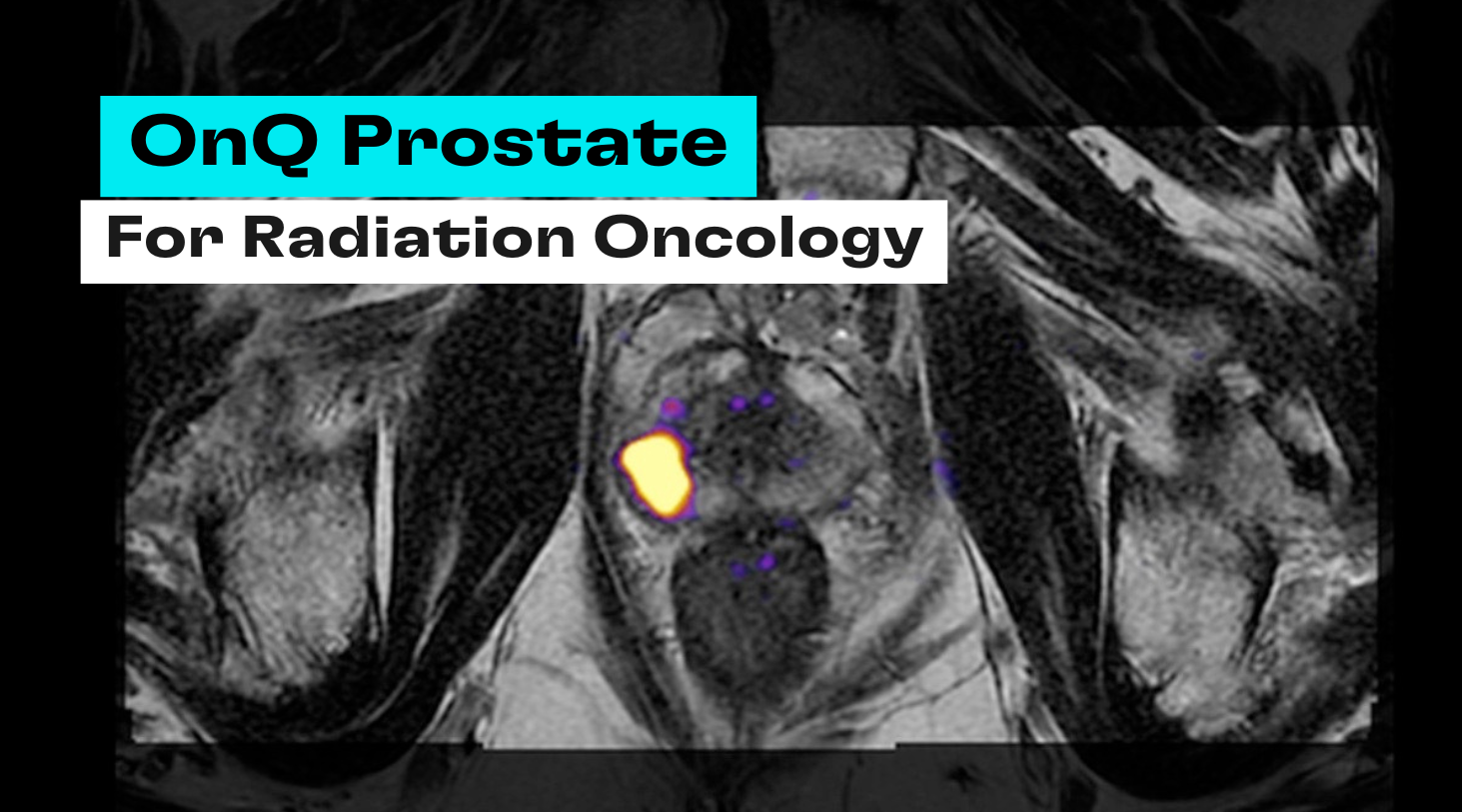
OnQ Prostate is an FDA‑cleared software solution that uses Restriction Spectrum Imaging (RSI), a patented diffusion MRI technique. RSI focuses on intracellular restricted diffusion and provides clearer, more accurate images of prostate tissue, improving identification of aggressive cancer cells1. Compared with standard diffusion‑weighted imaging/apparent diffusion coefficient approaches, RSI offers better tissue characterization and enhances the conspicuity of suspicious lesions, enabling reliable differentiation between benign and malignant tissues and boosting the sensitivity and specificity of prostate cancer detection2. By delivering clear, easy‑to‑interpret images, OnQ Prostate helps bridge the gap between radiologists and referring physicians and supports collaborative decision‑making in biopsy and therapy targeting.
To enable standardized billing and outcomes tracking for emerging quantitative imaging tools like OnQ Prostate, the American Medical Association (AMA) introduced two Category III CPT® codes—0648T and 0649T. These temporary codes, which took effect 1 July 2021, allow providers to report quantitative magnetic resonance services and support the collection of utilization data needed for potential future Category I coding.
Understanding Category III CPT® Codes
Category III codes are distinct from the typical Category I codes. They are temporary codes used to track emerging technologies, services and procedures. The AMA notes that Category III codes facilitate data collection for emerging services and should be reported instead of unlisted Category I codes when an appropriate Category III code exists. The data collected may be used to substantiate widespread usage by the AMA, which will justify a Category I (permanent) CPT code.
Importance of Data Collection
Consistent reporting of Category III codes benefits both providers and patients. The AMA designed these codes to capture real‑world data on adoption, utilization and outcomes. This evidence helps specialties and payers evaluate new technologies and may support the transition to a permanent Category I code in the future. For OnQ™ Prostate, thorough documentation and accurate coding contribute to building the evidence base for routine coverage of quantitative neuroimaging tools.
Code Descriptions
The codes applicable for OnQ Prostate describe quantitative magnetic resonance for analysis of tissue composition (e.g., fat, iron or water content). They apply to a single organ and include multiparametric data acquisition, data preparation, transmission, interpretation and reporting. The key difference is whether the service is performed with or without a diagnostic MRI:
0648T: Quantitative magnetic resonance for analysis of tissue composition (eg, fat, iron, water content), including multiparametric data acquisition, data preparation and transmission,interpretation and report, obtained without diagnostic MRI examination of the same anatomy (eg, organ, gland, tissue, target structure) during the same session; single organ
0649T: Quantitative magnetic resonance for analysis of tissue composition (eg, fat, iron, water content), including multiparametric data acquisition, data preparation and transmission, interpretation and report, obtained with diagnostic MRI examination of the same anatomy (eg, organ, gland, tissue, target structure); single organ – list seperately in addition to code for primary procedure
Practical Reimbursement Guidance
- Verify payer policies – Because Category III codes do not guarantee payment, each payer may have different coverage criteria. Urologists and radiology practices should check with Medicare contractors and private insurers to determine whether the service is covered.
- Document medical necessity – Reimbursement decisions are based on clinical evidence and necessity rather than the CPT code category. Practices should document why quantitative MRI was needed (e.g., improving characterization of suspicious lesions) and ensure that the diagnostic MRI and quantitative analysis are medically indicated.
- Use appropriate workflow – Obtain any required prior authorization and clarify whether the code should be submitted for tracking only or for billing purposes.
- Build the evidence – Consistent reporting of 0648T and 0649T helps generate utilization and outcomes data, which is essential for evaluating the technology and potentially obtaining broader coverage. Providers play an important role in demonstrating the clinical value of quantitative MRI analysis.
Support for OnQ™ Prostate Users
Implementing category III codes can be complex. Cortechs.ai, the vendor behind OnQ Prostate, offers a Comprehensive Reimbursement Guide and toolkit. The guide walks practices through confirming local payer policies, setting up internal workflows, documenting medical necessity and managing denials/appeals. To request the guide or ask questions about integrating codes 0648T/0649T into practice, contact the company’s reimbursement team at reimbursementsupport@cortechs.ai. Consistent and appropriate reporting will help build the real‑world evidence needed to advance coverage for quantitative imaging tools.
Conclusion
OnQ Prostate leverages RSI‑MRI technology to provide automated quantitative analysis of prostate tissue, aiding in the detection of clinically significant cancer. The AMA’s Category III CPT codes 0648T and 0649T enable providers to report these emerging services and contribute to the evidence base. Because Category III codes are temporary and reimbursement varies by payer, practices should verify coverage, document medical necessity and use the codes accurately. Engaging with reimbursement support resources can help ensure compliant implementation and facilitate the transition from temporary tracking codes to broader reimbursement pathways.
References
[1] Kevin C. McCammack et al., “In Vivo Prostate Cancer Detection and Grading Using Restriction Spectrum Imaging‑MRI,” Prostate Cancer and Prostatic Diseases 19, no. 2 (2016): 168–173, https://doi.org/10.1038/pcan.2015.61
[2] Ghiam Yamin et al., “Voxel‑Level Radiologic–Pathologic Validation of Restriction Spectrum Imaging Cellularity Index with Gleason Grade in Prostate Cancer,” Clinical Cancer Research 22, no. 11 (2016): 2668–2674, https://doi.org/10.1158/1078‑0432.CCR‑15‑2429