Watch the full Applied Radiology video here
Disease Modifying Therapies
NeuroQuant, Cortechs.ai’s advanced AI-driven brain volumetric analysis software, is elevating Alzheimer’s disease (AD) care, according to Suzie Bash, MD, a Medical Director at RadNet and Chief Medical Officer at Cortechs.ai. NeuroQuant delivers accurate, AI-driven volumetric analysis of brain structures over time, providing substantial benefits for diagnosing and monitoring neurodegenerative conditions. With its commercial debut in 2002, Cortechs.ai became the pioneer of quantitative volumetric MRI (QMRI), revolutionizing diagnostic precision for neurodegenerative diseases.
“The AI tool identifies and labels anatomic brain structures, then calculates their volumes to enable longitudinal tracking of disease progression,” Dr. Bash explained to Applied Radiology at RSNA 2024.
Clinical Impact in Alzheimer’s Disease
The advent of disease-modifying therapies (DMTs) such as lecanemab (Leqembi®) and donanemab (Kisunla™) has transformed the treatment landscape for Alzheimer’s disease, offering new hope to patients, says Dr. Bash. These advancements bring unique imaging challenges and opportunities. Radiologists are now experiencing a substantial increase in MRI brain, NeuroQuant, and amyloid PET volumes.
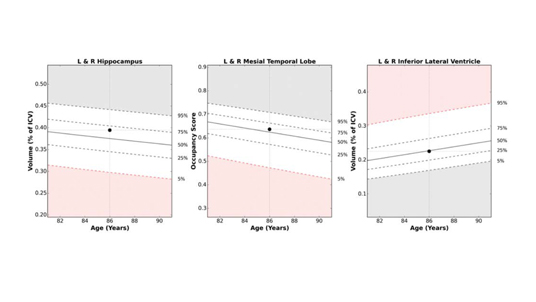
NeuroQuant enables detailed volumetric assessments of brain substructures associated with memory loss, including the hippocampus, entorhinal cortex, and lobar cortices. By comparing these measurements to a robust, age- and gender-matched normative database, the software aids in differentiating Alzheimer’s disease from other neurodegenerative conditions such as frontotemporal dementia. Automated analysis not only reduces inter-reader variability but also requires no additional image acquisition time, making it a cost-effective diagnostic and surveillance tool, says Dr. Bash.
In the DMT era, NeuroQuant has introduced novel capabilities with its recent v5.0 release. It now supports pre-treatment DMT eligibility screening on the baseline MRI through detection and quantification of microhemorrhages on gradient-echo (GRE) or susceptibility-weighted imaging (SWI). Additionally, NeuroQuant can aid in the evaluation of treatment-related side effects, namely amyloid-related imaging abnormalities (ARIA). Specifically, the software can detect intracranial blood products and evaluate for interval change, an application useful in surveillance for ARIA-H (microhemorrhage or superficial siderosis). It can also identify and quantitate new areas of FLAIR hyperintensity, which is useful in surveillance for ARIA-E (edema or sulcal effusion). These features can assist radiologists in radiographic grading of ARIA, facilitating comprehensive monitoring of therapy effects.1
Early Diagnosis
“Early diagnosis is critical for optimizing the therapeutic benefit of DMTs,” emphasizes Dr. Bash. She further highlights that tau deposition and brain atrophy co-occur at the onset of cognitive decline, contrasting with amyloid deposition, which may precede symptoms by decades. Since Tau PET is not yet reliably reimbursed in the clinical setting, NeuroQuant can serve as a vital subclinical biomarker, helping to identify patients in the earliest stage of disease, such as those with early cognitive decline and a positive amyloid PET, but with preserved brain volumes on QMRI, says Dr. Bash.
The importance of early diagnosis was underscored in the CLARITY AD trial, which demonstrated that initiating DMTs in the earliest stages of Alzheimer’s disease resulted in cognitive improvement for 60% of patients and prevented cognitive decline in 76% at 18 months.
Streamlining Workflow and Enhancing Precision
NeuroQuant integrates seamlessly into clinical workflows, offering rapid, cloud-based processing with results available in under six minutes. According to Dr. Bash, the software’s user-friendly reports eliminate the need for post-processing by radiologists, enhancing efficiency. “Using NeuroQuant also offers a referral advantage,” Dr. Bash noted. “In my 19 years of experience, no referring physician who started using NeuroQuant has stopped.”
Cortechs.ai recently achieved significant progress in reimbursement, with several Medicare Administrative Contractors now consistently covering NeuroQuant under newly implemented vendor-neutral CPT III codes.2 This development provides a positive return on investment for imaging enterprises adopting the software.
Transforming Precision Medicine in Neurodegenerative Disease
With nearly three decades of expertise and millions of exams conducted, Cortechs.ai continues to lead the evolution of quantitative imaging. “This is truly precision medicine,” states Dr. Bash, highlighting NeuroQuant’s ability to reduce reader subjectivity by leveraging a normative database for standardized, accurate, and reproducible reports. This standardization is crucial for gaining acceptance among referrers and improving diagnostic consistency.
Kyle Frye, CEO of Cortechs.ai, emphasizes the company’s mission to empower healthcare providers with advanced tools for both diagnosis and treatment monitoring. “We’re not just part of the diagnostic pathway but also part of the treatment journey,” Frye explains.