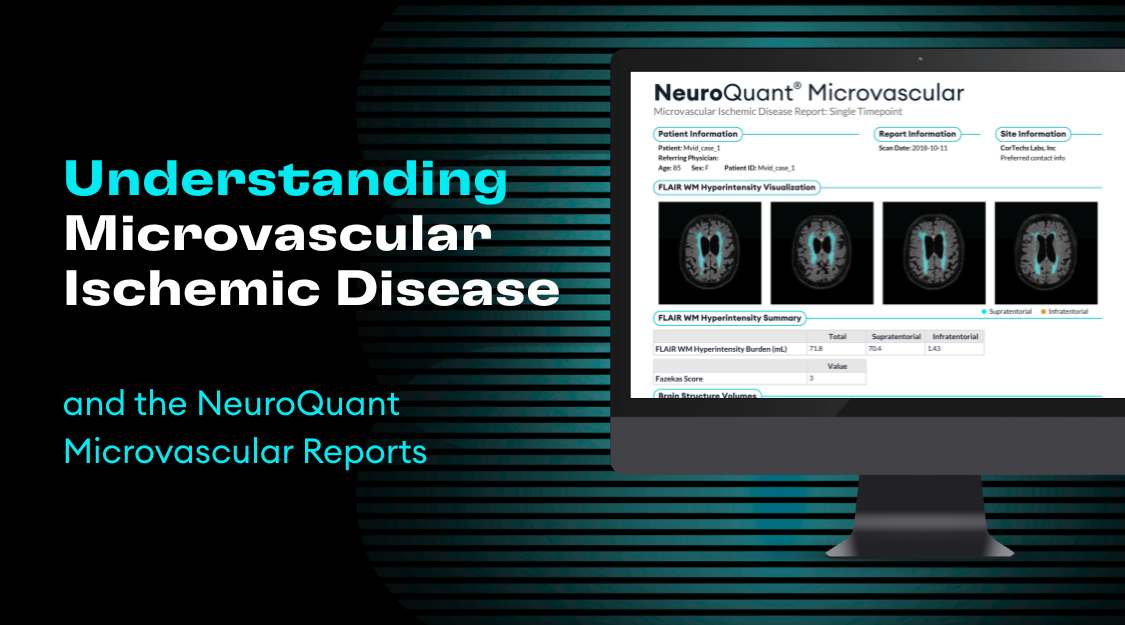
The recently updated “Alzheimer’s Disease Anti-Amyloid Immunotherapies: Imaging Recommendations and Practice Considerations for ARIA Monitoring” 2024 guidelines for monitoring Amyloid-Related Imaging Abnormalities (ARIA) in Alzheimer’s disease patients undergoing anti-amyloid immunotherapy reflect significant advancements compared to earlier versions (1). The newer recommendations are shaped by the full FDA approval from the Centers for Medicare & Medicaid Services (CMS) coverage of therapies like lecanemab (leqembi) and donanemab (kisunla).
The updated guidelines emphasize the practical aspects of radiology, particularly in implementing standardized imaging protocols and enhancing communication between radiologists and the broader care team. This contrasts with the older guidelines, which primarily focused on the pathophysiological mechanisms of ARIA and its detection during clinical trials, providing a more theoretical framework that lacked the practical, clinical implementation guidance seen in the 2024 update.
One of the most notable advancements in the 2024 article is the integration of artificial intelligence (AI) tools in the monitoring and surveillance of ARIA. AI assistance is highlighted as a potential game-changer in the radiology field, particularly for its role in improving the accuracy and consistency of ARIA detection. The use of AI can help automate the identification of microhemorrhages and edema associated with ARIA, which are often subtle and challenging to detect. This is especially beneficial given the expected increase in imaging volumes due to broader access to anti-amyloid therapies. By reducing inter-rater variability and enhancing the precision of ARIA detection, AI tools contribute to more reliable monitoring, ultimately supporting better patient outcomes.
The 2024 guidelines also expand on the clinical and practical considerations for radiologists, reflecting the lessons learned from earlier studies. While the older version provided a foundational understanding of ARIA’s radiological, biological, and clinical characteristics, the updated guidelines focus more on real-world application. This includes detailed protocols for different clinical scenarios, such as baseline evaluations, routine monitoring of asymptomatic patients, and the management of symptomatic ARIA. These scenarios are accompanied by specific MRI protocols and recommendations for imaging standardization across different practice settings, ensuring that the increased demand for radiological services can be met effectively.
In conclusion, the evolution from the earlier ARIA guidelines to the 2024 update represents a shift from theoretical knowledge to practical application, driven by the latest advancements in treatment options and diagnostic technologies.
The inclusion of AI as a tool for ARIA surveillance such as NeuroQuant for ARIA (FDA-cleared) exemplifies this shift, providing radiologists with the means to manage the increasing complexity and volume of cases with greater confidence and accuracy. This progress underscores the importance of ongoing updates and refinements in clinical guidelines to keep pace with advancements in both therapeutic and diagnostic arenas.
Citations:
- Cogswell, Petrice M., et al. “Alzheimer’s Disease Anti-Amyloid Immunotherapies: Imaging Recommendations and Practice Considerations for ARIA Monitoring.” American Journal of Neuroradiology, vol. 45, no. 8, 2024, pp. 1234-1256. Published online 23 Aug. 2024, https://doi.org/10.3174/ajnr.A8469.
- Cogswell, Petrice M., et al. “Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice.” American Journal of Neuroradiology, vol. 43, no. 9, 2022, pp. E19-E35. Published August 25, 2022, https://doi.org/10.3174/ajnr.A7586.
- Hampel, Harald, et al. “Amyloid-Related Imaging Abnormalities (ARIA): Radiological, Biological, and Clinical Characteristics.” Brain, vol. 2023, Oxford University Press, https://doi.org/10.1093/brain/awad188. Accessed 20 June 2023.