By
Cortechs.ai
2 mins
Cortechs.ai Announces NeuroQuant 3.1 Product Release
Redesigned reports for improved clinical volumetric analysis
(SAN DIEGO) April 28, 2021 – Cortechs.ai today announced that NeuroQuant version 3.1 is available to new and existing customers. NeuroQuant, the first FDA 510(k) cleared medical device software for quantitative analysis from MRI, provides fast and accurate brain image analysis.
LesionQuant, a module of NeuroQuant often used clinically for the diagnosis and assessment of multiple sclerosis (MS), is now called NeuroQuant MS as of the 3.1 release. NeuroQuant report names were modified slightly to better reflect their typical clinical uses. The reports now available are:
- NeuroQuant Dementia: Age-Related Atrophy Report
- NeuroQuant Pediatrics: Brain Development Report
- NeuroQuant Seizure: Hippocampal Asymmetry Report
- NeuroQuant MSA: Multi-Structure Atrophy Report
- NeuroQuant TBI: Triage Brain Atrophy Report
- NeuroQuant Morphometry: General Morphometry Report
- NeuroQuant Custom: Custom Volumetric Report
- NeuroQuant MS: FLAIR Lesion and Atrophy Report
- NeuroQuant MS: FLAIR Lesion and Atrophy Report Plus
Data available on each NeuroQuant report remains the same, with the exception of NeuroQuant Dementia: Age-Related Atrophy report which now includes the entorhinal cortex. New features of the NeuroQuant MS reports include the addition of T1 hyperintensities’ volumes, the addition of the lesion burden calculation by hemisphere, and the option of including automatic lesion counts on the report. Additionally, all NeuroQuant products available for clinical use now support parallel imaging for 1.5T MRI scanners.
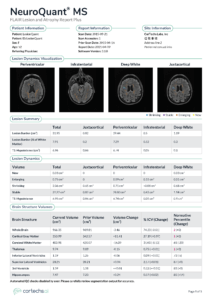
NeuroQuant MS: FLAIR and Atrophy Report is now a single page which includes:
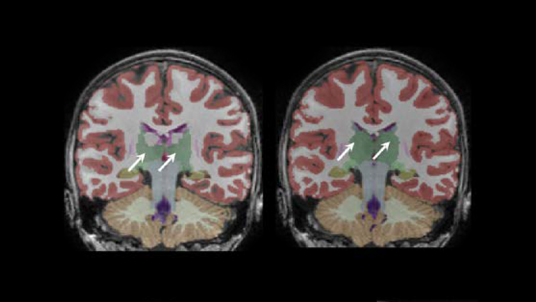
- Lesion visualization overlays with MRI slices which have the most lesions for each region
- Lesion counts, volumes, and burden for each region
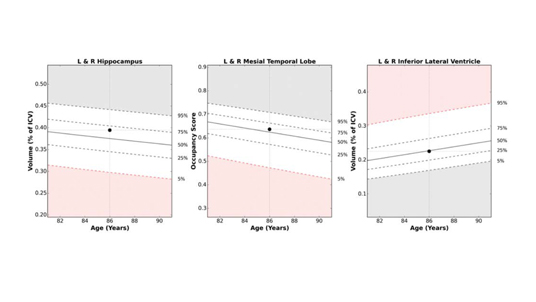
- Brain structure volumes, percent of intracranial volume, and normative reference percentiles
NeuroQuant MS: FLAIR and Atrophy Report Plus is now a 5-page report which includes:
- Lesion visualization overlays with MRI slices which have the most lesions for each region
- Lesion counts, volumes, and burden for each region
- Brain structure volumes, percent of intracranial volume, and normative reference percentiles
- Lesion summary of written text for lesions found
- Lesion location section providing counts, volumes, and burden in a visual form
- Lesion hemisphere section providing lesion counts by hemisphere and burden by hemisphere
- Individual lesion visualization overlays
- Brain structure visualization overlays
- Normative reference charts
- Interpretation summary describing definitions of each section
For more information about NeuroQuant 3.1 changes visit: https://www.cortechs.ai/.
Current customers, both cloud and installed, will not be automatically migrated to NeuroQuant 3.1 and must coordinate with an account manager to activate this version.
The reports have also been redesigned to reflect the rebranding from CorTechs Labs to Cortechs.ai, which was announced 1/1/21. This version was released without modifications to the products’ volumetric algorithm, and volumetric measurements of this release are identical to those computed in the 3.0.2 and 3.0.0 releases.
About Cortechs.ai
Cortechs.ai is the leader in radiology AI applications, using cutting-edge advances in medical imaging to revolutionize disease screening and early detection so patients can enjoy longer, healthier lives. The company develops and markets breakthrough medical device software that quantifies and tracks neurodegenerative diseases and assists in the detection of clinically significant cancer. Cortechs.ai’s industry-leading brain imaging analysis provides radiologists, neurologists, oncologists, and clinical researchers worldwide with a convenient and cost-effective means to quantify brain structures to help assess neurological conditions, such as Alzheimer’s disease, epilepsy, multiple sclerosis, brain trauma, and brain development abnormalities. The company has FDA-cleared products for use in the diagnosis and follow up of neurodegenerative and traumatic brain conditions, as well as prostate cancer. Please visit www.cortechs.ai for further information and follow us on Twitter, LinkedIn, and Facebook.
Contact: Travis Foegler
Phone: +1 (619) 450-9094
Email: tfoegler@cortechslabs.com
Share